Explain the Major Differences Between Covalent and Ionic Bonding
Generally ionic bonds are stronger than covalent bonds. Complete step by step answer.
What S The Difference Between An Ionic Bond And A Covalent Bond Quora
Prefixes Do not criss-cross.
. 7 rows Ionic and covalent bonds are fundamentally different in the way they are formed. A covalent bond is formed due to mutual sharing of electrons between two atoms of a non-metal. Bond Energy is higher than metallic.
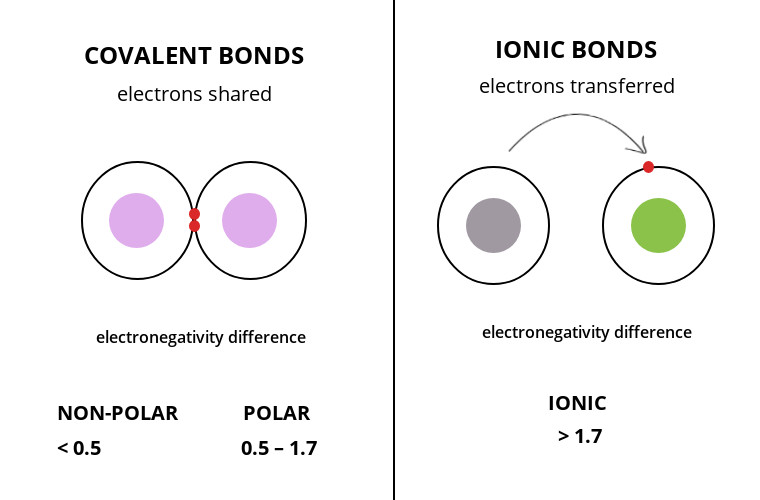
Ionic is losing and gaining electron s Covalent is sharing the same electron s Explanation. 100 2 ratings C Ionic bonding is the complete transfer of Valence electrons between atomsit is a type of chemical bond that generates two oppositely charged ionsIn ionic bondsthe metal loses electrons to become a positively charged cation. -In an ionic bond the atoms are bound together by the attraction between oppositely-charged ions.
9 rows Difference Between Ionic Covalent and Metallic bonds The attractive force which holds together. What is the main difference between an ionic and covalent bond quizlet. See the answer Show transcribed image text Expert Answer 222aThe main differences between the various forms of primary bonding are.
Lets differentiate between an ionic bond and a covalent bond Read More. The polar covalent bond or heteropolar covalent bond occurs between atoms of different elements but the two atoms must have an electronegativity difference of less than 19. Covalent bond involves the sharing of electrons while metallic bonds have strong attractions and ionic bonds involve the transferring and accepting of electrons from the valence shell.
Difference Between Ionic Covalent and Metallic Bonds Definition. Covalent bonds are formed in Methane and Ionic bonds are formed in sodium chloride. The type of bond determines the structure stability and properties of a substance.
Ionic transfers electrons and covalent shares electrons. The making of ceCl2 leads to a formation of a. Dinitrogen Hexafluoride Between two NONMETALS Sharing of Electrons Change endings of the last element to.
Ionic - One atom loses and electron the other gains one and two oppositely charged ions are produced which are attracted to each other. When writing a covalent molecule use. Covalent bonding happens between atoms of little completely totally different in electronegativity.
The ionic bond occurs when the difference in electronegativity between the two elements that intend to bond is greater than 19. Ionic bonds are forces that hold together electrostatic forces of. This mutual sharing of electrons between the two non-metal species leads to the formation of a covalent bond.
What is the difference between ionic and covalent compounds. The difference between naming ionic covalent compounds Covalent Bonds Example. Formed by transfer of an electron from one atom to another.
What is the name for N2F6. Tap card to see definition. Ionic bonding happens between atoms of good in electronegativity.
Now try to figure out the properties of bonds found in these compounds. The compound has the low melting point. The adhering property of an atom in order to arrange themselves in a most stable pattern by filling their outermost electrons orbit.
Formed by a sharing of electrons between two atoms. Ionic bonds dissociate in water to result in ions whereas covalent. Ionic--there is electrostatic attraction between oppositely charged ions.
Ionic bonds exist between a metal and non-metal while covalent bond exists between non-metals. Ionic bonds are formed when two atoms have a large difference in their electronegativity values. Click card to see definition.
The rate of reaction of covalent bonds is comparatively low whereas that of an ionic bond is comparatively instantaneous. What is the difference between Covalent and Ionic Bonds. Here participating atoms have.
Chemical bonds can affect the stability of a compound. Helmenstine Anne Marie PhD. The three main types of chemical bonds are ionic bonds covalent bonds and metallic bonds.
A compound has the high melting point. -In a covalent bond the atoms are bound by shared electrons. Covalent--there is electron shari.
Covalent bonds are poor conductors whereas ionic bonds are good conductors in a molten state. Bond Energy is higher than metallic bonds. Click again to see term.
Covalent bonds are non-conductors whereas ionic bonds are conductors. Ionic bonds require extreme melting and a boiling degree in case of ionic bonding. What are Ionic Compounds.
View the full answer. The difference between an ionic and a covalent bond is that a covalent bond is formed when two atoms share electrons. Covalent - A shared pair of electrons resulting in both atoms having full outer shells.
This leads to the differences in their physical properties. 7 rows An ionic bond essentially donates an electron to the other atom participating in the bond. Ionic Bond Definition ThoughtCo Feb.
In covalent bonding two or more atoms share electrons to satisfy the octet rule. Those with weak chemical bonds are usually less stable. The difference between ionic and covalent bond is that ionic bonds occur between atoms having very different electronegativities whereas covalent bonds occur between atoms with similar or very low electronegativity differences.
Ionic bonds are electrostatic forces arising between negative and positive ions.
Difference Between Covalent And Ionic Bonds

Difference Between Ionic And Covalent Bonds Compare The Difference Between Similar Terms

15 Major Difference Between Covalent And Ionic Bonds With Table Core Differences
No comments for "Explain the Major Differences Between Covalent and Ionic Bonding"
Post a Comment